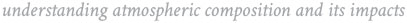LABORATORY FACILITIES
 Jesse Kroll’s laboratory is located in the Parsons Laboratory for Environmental Science and Engineering. A major feature of the laboratory is an environmental chamber for the study of gas-phase chemistry and secondary organic aerosol formation. The chamber consists of a temperature-controlled room (10C-40C), containing fluorescent blacklights (centered at 350 nm, 1.8 kW total) and an inner chamber (7.5 m3) in which the reactions are run. A number of analytical instruments are available for the measurement of the chemistry within the chamber, including a high-resolution time-of-flight aerosol mass spectrometer, another high-resolution mass spectrometer for the measurement of semivolatile and intermediate-volatility organics; a filter sampler for offline chemical analysis; a scanning mobility particle sizer; a GC-FID; an NO/NOx chemiluminescence monitor; and an O3 monitor. Several other smaller reactors (a chamber and two flow tubes) are currently under construction.
Jesse Kroll’s laboratory is located in the Parsons Laboratory for Environmental Science and Engineering. A major feature of the laboratory is an environmental chamber for the study of gas-phase chemistry and secondary organic aerosol formation. The chamber consists of a temperature-controlled room (10C-40C), containing fluorescent blacklights (centered at 350 nm, 1.8 kW total) and an inner chamber (7.5 m3) in which the reactions are run. A number of analytical instruments are available for the measurement of the chemistry within the chamber, including a high-resolution time-of-flight aerosol mass spectrometer, another high-resolution mass spectrometer for the measurement of semivolatile and intermediate-volatility organics; a filter sampler for offline chemical analysis; a scanning mobility particle sizer; a GC-FID; an NO/NOx chemiluminescence monitor; and an O3 monitor. Several other smaller reactors (a chamber and two flow tubes) are currently under construction.

Ronald Prinn’s laboratory is located in the Department of Earth, Atmospheric and Planetary Sciences’ Green Building. The laboratory focuses on high-frequency on-site measurements of the major atmospheric gases involved in climate change and ozone-layer depletion. The laboratory assembles and deploys in the field instruments for the AGAGE global network, for individual doctoral thesis work (e.g., recently Drs. Kat Potter, Laura Meredith, and Anita Ganesan) and for the recent development of high-frequency measurements of isotopic composition of gases (see below).

Instrument to measure isotopomer ratios of tropospheric nitrous oxide (N2O, showing Arerodyne’s mid-IR spectroscopy (right), and preconcentration module developed at MIT.
Shuhei Ono and Ronald Prinn, in cooperation with Aerodyne Research Inc. have developed an instrument for on-site monitoring of tropospheric nitrous oxide isotopomer ratios (15N14N16O/14N15N16O/14N14N16O). The instrument utilizes Aerodyne Research’s quantum cascade mid-infrared laser spectroscopy combined with liquid-nitrogen free preconcentration unit used for AGAGE network. The instrument measures isotopomer ratios at every 20 min at ca. 0.2 ‰ precision (2s) fully autonomously. It is currently deployed at the Green Building to analyze air sampled from the roof of the building. The instrument will be deployed at one of the AGAGE stations in early 2013. This will be used to better quantify the source and sink strengths of toropospheric nitrous oxide.

Shown above is a vacuum manifold we use to fluorinate samples to SF6 that is introduced to a gas-source isotope ratio mass-spectrometer.
Shuhei Ono’s group uses isotope ratio mass-spectrometer (MAT 253) for high precision measurements of four sulfur isotope abundances (32S, 33S, 34S, 36S). We calibrate isotope fractionations for some key sulfur photochemical reactions. The lab also hosts four gas chromatography instruments for analyses of various trace gases (e.g., H2, CO, CH4, N2O, CO2)